Formaldehyde as a Precursor to Complex Biomolecules on Mars
In 2013, NASA's Curiosity rover detected organic compounds in Martian rock samples, prompting further investigation into their implications for the planet's history. A particularly intriguing aspect of these compounds is their unusual carbon isotopic ratios, notably a marked depletion in carbon-13 (13C), as well as a significant variability in this depletion between samples.
Now, researchers Tohoku University have managed to develop models to simulate the isotopic composition of carbon in these compounds over time, considering various factors such as CO and CO2 concentrations, atmospheric pressure, volcanic outgassing and solar influence. The study particularly focuses on the evolution of these ratios within formaldehyde (H2CO).
Members of this research team have previously demonstrated that photochemical reactions in the young Martian atmosphere could continually facilitate the formation of H2CO, making it a key player in understanding the history of the planet. This molecule is highly reactive and once in the water, could further react to produce sugars, amino acids, and other complex organic materials.
Mars’ atmosphere has undergone dramatic evolution throughout its lifespan, likely initially possessing a thick atmosphere rich in gases such as carbon dioxide (CO2), carbon monoxide (CO), and hydrogen (H2). Their initial atmospheric parameters incorporate molar fractions of 30%, 69% and 1% respectively.
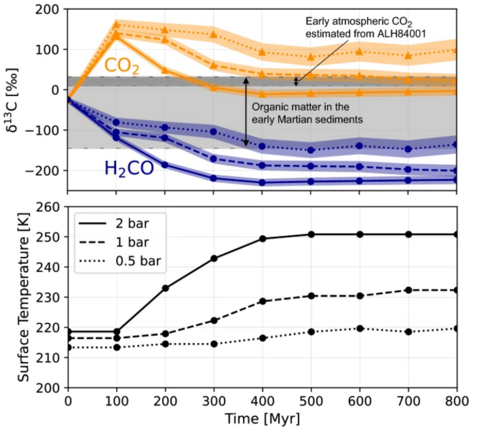
Their models identify that photochemical dissociation of CO2 leads to the fractionation of different Carbon isotopes. Due to the increased mass of molecules containing 13C, they are more stable and less likely to be dissociated. This leads to an increased ratio of CO2 containing this isotope. Meanwhile, molecules containing 12C are broken up into C and CO, the latter of which is able to react to form formaldehyde. Consequently, the formaldehyde and any organic compounds derived from it would share this isotopic depletion.
While H2CO showed a decline in 13C, CO2 itself became enriched in this isotope over time to the extent that they managed to reproduce the values in ancient Martian meteorites like ALH84001. This suggests that the processes transforming CO2 into organic matter also played a role in creating the carbonates present within meteorite samples.
If formaldehyde played such a significant role in the composition of the Martian atmosphere, it indicates that the planet possessed the necessary ingredients form complex biomolecules, before transitioning to its current cold, arid state. The team emphasise a need to better constrain their models with Carbon isotope ratios fixed at different historical epochs. The team avidly await the samples that will be collected by NASA’s MSR, and ESA’s MMX missions, both of which aim to retrieve samples from Phobos, the larger of Mars’ two moons.
--
Journal Source: S. Yoshida et al, Stable carbon isotope evolution of formaldehyde on early Mars, Vol 14, Scientific Reports, (2024), DOI: https://doi.org/10.1038/s41598-024-71301-w
Cover Image Credit: Emirates Mars Mission
